Hepatitis Testing Guidelines
Health Care
The League of Extraordinary Providers
Harm Reduction
Elimination Tools
Hepatitis Treatment Guidelines
Additional Resources
Oregon Viral Hepatitis Collective

Hepatitis B
CDC Recommendations for Routine Testing and Follow-up for Chronic Hepatitis B Virus (HBV) Infection – Oregon Health Plan follows these recommendations
| Population | Recommendation | |
|---|---|---|
| Testing | Vaccination/Follow-up | |
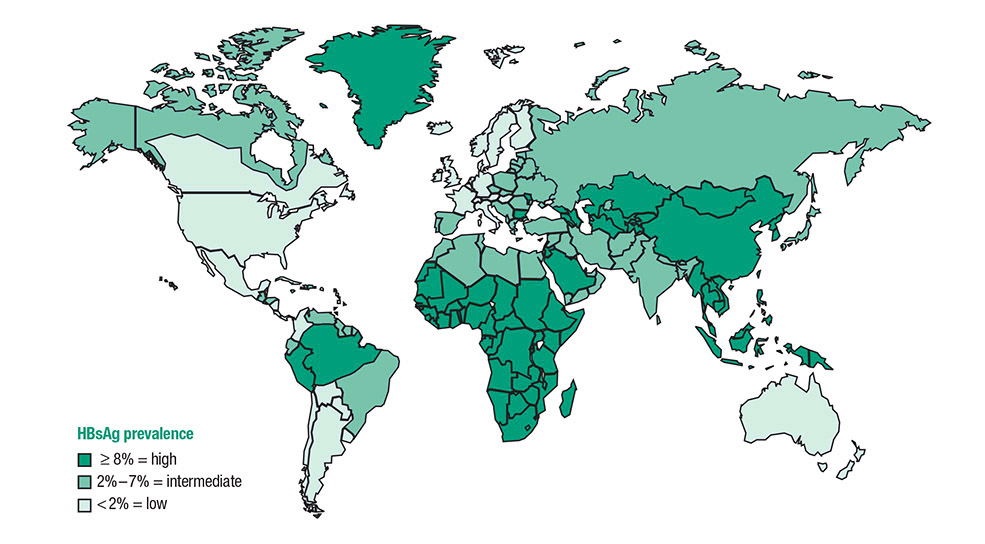
| Persons born in regions of high and intermediate HBV endemicity (HBsAg prevalence 2%) | Test for HBsAg, regardless of vaccination status in their country of origin, including – immigrants – refugees – asylum seekers – internationally adopted children |
If HBsAg-positive, refer for medical management. If negative, assess for on-going risk for hepatitis B and vaccinate if indicated. |
| US born persons not vaccinated as infants whose parents were born in regions with high HBV endemicity ( 8%) | Test for HBsAg regardless of maternal HBsAg status if not vaccinated as infants in the United States. | If HBsAg-positive, refer for medical management. If negative, assess for on-going risk for hepatitis B and vaccinate if indicated. |
| Population | Recommendation | |
|---|---|---|
| Testing | Vaccination/Follow-up | |
| Injection-drug users | Test for HBsAg, as well as for anti-HBc or anti-HBs to identify susceptible persons. | First vaccine dose should be given at the same visit as testing. Susceptible persons should complete a 3-dose hepatitis B vaccine series to prevent infection from ongoing exposure. |
| Men who have sex with men | Test for HBsAg, as well as for anti-HBc or anti-HBs to identify susceptible persons. | First vaccine dose should be given at the same visit as testing. Susceptible persons should complete a 3-dose hepatitis B vaccine series to prevent infection from ongoing exposure. |
| Persons needing immunosuppressive therapy, including chemotherapy, immunosuppression related to organ transplantation, and immunosuppression for rheumatologic or gastroenterologic disorders | Test for all markers of HBV infection (HBsAg, anti-HBc, and anti-HBs). | Treat persons who are HBsAg-positive. Monitor closely persons who are anti-HBc positive for signs of liver disease. |
| Persons with elevated ALT/AST of unknown etiology | Test for HBsAg along with other appropriate medical evaluation. | Follow-up as indicated. |
| Donors of blood, plasma, organs, tissues, or semen | Test for HBsAg, anti-HBc, and HBV-DNA as required. | |
| Hemodialysis patients | Test for all markers of HBV infection (HBsAg, anti-HBc, and anti-HBs). Test vaccine nonresponders monthly for HBsAg. HBsAg-positive hemodialysis patients should be cohorted. |
Vaccinate against hepatitis B to prevent transmission and revaccinate when serum anti-HBs titer falls below 10mIU/mL. |
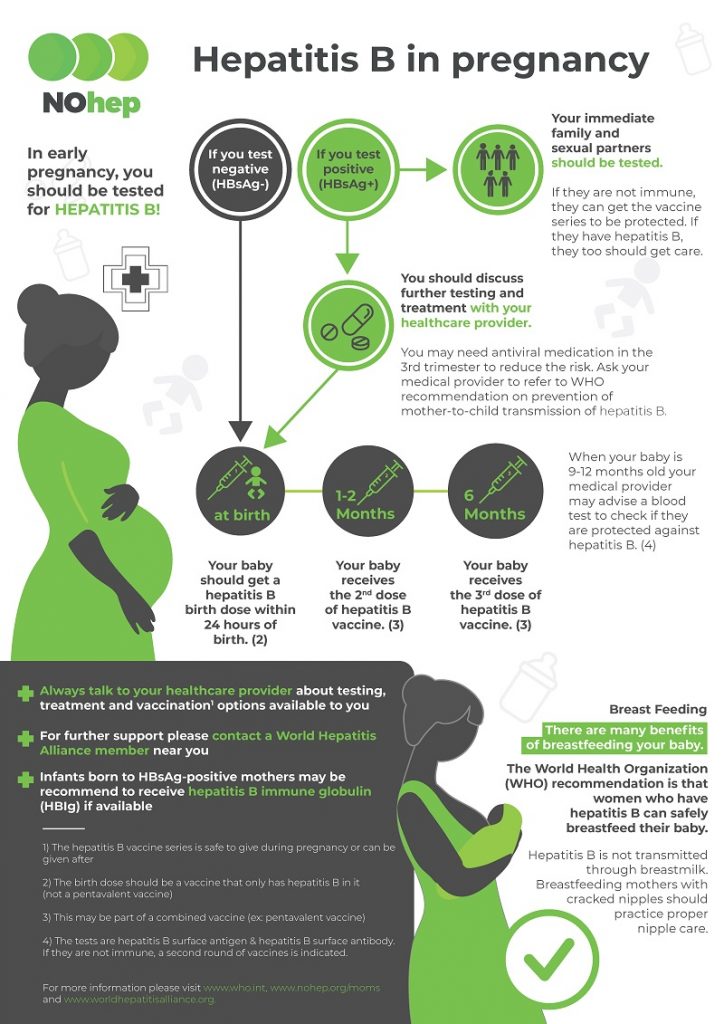
| All pregnant women | Test for HBsAg during each pregnancy, preferably in the first trimester. Test at the time of admission for delivery if prenatal HBsAg test result is not available or if mother was at risk for infection during pregnancy. |
If HBsAg-positive, refer for medical management. To prevent perinatal transmission, infants of HBsAg-positive mothers and unknown HBsAg status mothers should receive vaccination and postexposure immunoprophylaxis in accordance with recommendations and within 12 hours of delivery. |
| Infants born to HBsAg-positive mothers | Test for HBsAg and anti-HBs 1–2 mos after completion of at least 3 doses of a licensed hepatitis B vaccine series (i.e., at age 9–18 months, generally at the next well-child visit to assess effectiveness of postexposure immunoprophylaxis). Testing should not be performed before age 9 months or within 1 month of the most recent vaccine dose. | Vaccinate in accordance with recommendations. |
| Household, needle-sharing, or sex contacts of persons known to be HBsAg positive | Test for HBsAg, as well as anti-HBc or anti-HBs to identify susceptible persons. | First vaccine dose should be given at the same visit as testing. Susceptible persons should complete a 3-dose hepatitis B vaccine series to prevent transmission from ongoing exposure. |
| Persons who are the sources of blood or body fluids resulting in an exposure (e.g., needlestick, sexual assault) that might require postexposure prophylaxis | Test source for HBsAg. | Vaccinate health care and public safety workers with reasonably anticipated occupational exposures to blood or infectious body fluids. Provide postexposure prophylaxis to exposed person if needed. |
| HIV-positive persons | Test for HBsAg, as well as for anti-HBc or anti-HBs to identify susceptible persons. | Vaccinate susceptible persons against hepatitis B to prevent transmission. |
Adapted from: Centers for Disease Control and Prevention. Recommendations for Identification and Public Health Management of Persons with Chronic Hepatitis B Virus Infection. MMWR 2008; 57 (No. RR-8).
Source: https://www.cdc.gov/hepatitis/hbv/HBV-RoutineTesting-Followup.htm
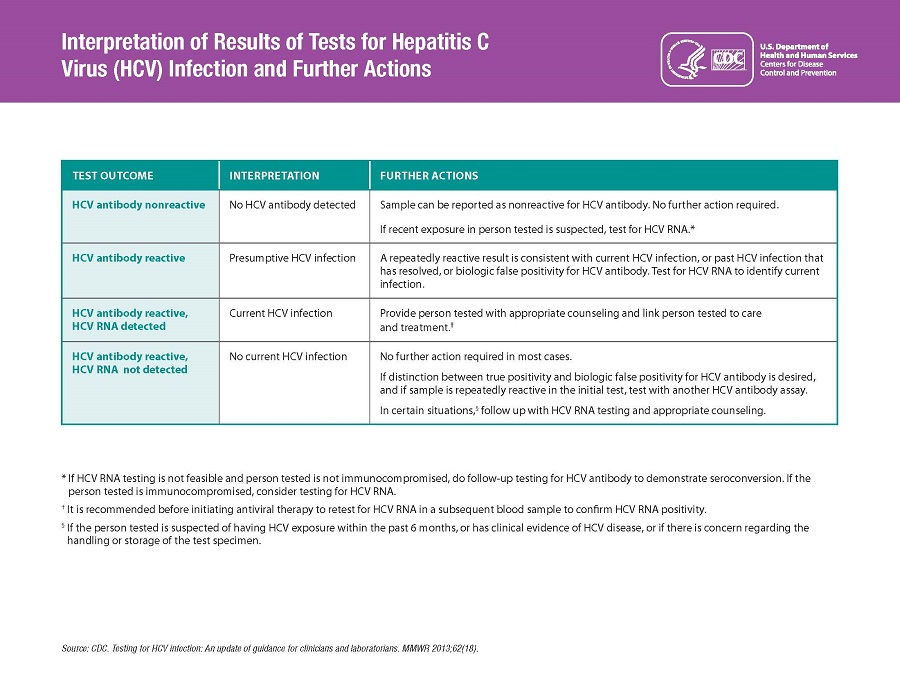
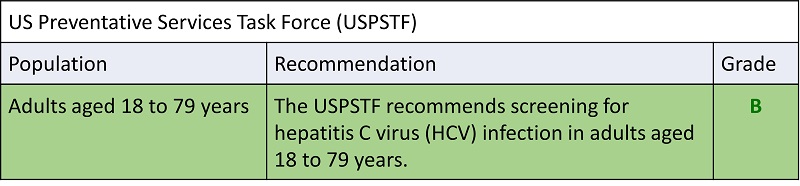
Hepatitis C
 CDC Recommendations for Hepatitis C Screening Among Adults in the United States –
CDC Recommendations for Hepatitis C Screening Among Adults in the United States –
Oregon Health Plan follows these recommendations
Universal hepatitis C screening:
-
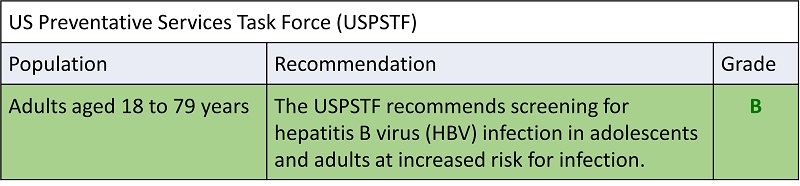
- Hepatitis C screening at least once in a lifetime for all adults aged 18 years and older, except in settings where the prevalence of HCV infection (HCV RNA‑positivity) is less than 0.1%*
- Hepatitis C screening for all pregnant women during each pregnancy, except in settings where the prevalence of HCV infection (HCV RNA‑positivity) is less than 0.1%*
One‑time hepatitis C testing regardless of age or setting prevalence among people with recognized conditions or exposures:
- People with HIV
- People who ever injected drugs and shared needles, syringes, or other drug preparation equipment, including those who injected once or a few times many years ago
- People with selected medical conditions, including:
- people who ever received maintenance hemodialysis
- people with persistently abnormal ALT levels
- Prior recipients of transfusions or organ transplants, including:
- people who received clotting factor concentrates produced before 1987
- people who received a transfusion of blood or blood components before July 1992
- people who received an organ transplant before July 1992
- people who were notified that they received blood from a donor who later tested positive for HCV infection
- Health care, emergency medical, and public safety personnel after needle sticks, sharps, or mucosal exposures to HCV‑positive blood pdf icon[PDF – 177 KB]
- Children born to mothers with HCV infection
Routine periodic testing for people with ongoing risk factors, while risk factors persist:
- People who currently inject drugs and share needles, syringes, or other drug preparation equipment
- People with selected medical conditions, including:
- people who ever received maintenance hemodialysis
Any person who requests hepatitis C testing should receive it, regardless of disclosure of risk, because many persons may be reluctant to disclose stigmatizing risks
*Determining prevalence: In the absence of existing data for hepatitis C prevalence, health care providers should initiate universal hepatitis C screening until they establish that the prevalence of HCV RNA positivity in their population is less than 0.1%, at which point universal screening is no longer explicitly recommended but may occur at the provider’s discretion.
Source: https://www.cdc.gov/hepatitis/hcv/guidelinesc.htm [last accessed 1/26/22]